Drinking water, including bottled water, usually contains small amounts of contaminants. However, the presence of contaminants does not necessarily mean that the water poses a health risk.
Some contaminants are regulated under the Safe Drinking Water Act while others are unregulated at the current time. More information on both regulated and unregulated contaminants is below.
EPA-Regulated Contaminants
EPA sets National Primary Drinking Water Standards for approximately 90 contaminants. DEQ adopts and enforces these same standards. Information on specific contaminants is available on EPA’s website.
Contaminants of Interest
EPA has finalized over 100 different health advisories for regulated contaminants and unregulated contaminants with associated health advisories of current public interest.
EPA’s information on drinking water contaminants and health advisories can be found on EPA’s website.
This accordion will not appear on the screen
Arsenic is a naturally occurring element found in the earth’s crust. Trace amounts are found in all living matter, including rocks, soil, water, air, plants, and animals.
Arsenic is a well-known chemical element used in the manufacturing of agricultural chemicals such as pesticides, weed killers, and rodenticides. It is also used to produce paints, dyes, metals, drugs, soaps, and semiconductors. Approximately 90% of industrial arsenic in the United States is used as a wood preservative.
Arsenic can be released into the environment through natural activities such as volcanic action, erosion, forest fires, or through human activities such as pesticide application, improper disposal of arsenic-containing waste chemicals, agricultural applications, mining, and smelting.
Arsenic Standard – EPA’s drinking water standard for arsenic is 10 parts per billion (ppb). The standard applies to all community water systems and non-transient non-community water systems in Idaho.
Arsenic and Drinking Water – Most arsenic in drinking water comes from natural rock formations. Water that encounters rock formations can dissolve arsenic and carry it into underground aquifers, streams, and rivers that may be used as drinking water sources. Arsenic deposited on the ground from industrial or agricultural uses tends to persist in the top few feet of soil and is not likely to have a significant impact on most aquifers. When dissolved in water, arsenic has no smell, taste, or color, even at high concentrations.
Health Impacts of Arsenic Exposure – Arsenic is associated with more than 30 different adverse health effects, including cardiovascular disease, diabetes mellitus, skin changes, nervous system damage, and various forms of cancer. Although a very high dose (60,000 micrograms) of arsenic can be lethal, the amount of arsenic in drinking water is very small, and any drinking water-related health effects are the result of prolonged exposure over time. People may have different responses to the same arsenic exposure depending on dose, duration, general health, age, and other factors. Reducing the amount of arsenic in drinking water will lessen exposure and reduce the risk of adverse health effects.
Incidence of Arsenic – Western states have higher arsenic levels as compared to the rest of the United States. Parts of the Midwest and New England also have areas where arsenic levels are elevated. While many areas may not have detected arsenic in their drinking water above 10 ppb, there may be geographic hot spots with higher levels of arsenic than in surrounding areas.
Arsenic is a problem in some parts of Idaho. Data compiled by the Idaho Department of Water Resources show that concentrations of arsenic in ground water are highest in the southwestern Idaho counties of Elmore, Gem, Owyhee, and Washington; Kootenai County in northern Idaho; and Jefferson County in eastern Idaho. Other counties have moderate or only trace amounts of arsenic in historic ground water samples.
All community water systems are required to include health information and arsenic concentrations in their annual drinking water Consumer Confidence Report to DEQ for water that exceeds 5 ppb.
Private well owners are advised to sample their well water and have it tested by a certified drinking water laboratory.
Additional Resources
- Chemical Contaminant Rule – Arsenic
- Quick Reference Guide to the Arsenic Rule
- Arsenic in Drinking Water (NRDC)
- Arsenic and Drinking Water
- An Evaluation of Potential Associations between Arsenic Concentrations in Ground Water and 2000 – 2004 Cancer Incidence Rates in Idaho by Zip Code
- Arsenic and Your Distribution System
Coliform bacteria are organisms that are present in the environment and in the feces of all warm-blooded animals and humans. Coliform bacteria will not likely cause illness. However, their presence in drinking water indicates that disease-causing organisms (pathogens) could be in the water system. Most pathogens that can contaminate water supplies come from the feces of humans or animals.
Role of Coliforms in Detecting Contamination – Testing drinking water for all possible pathogens is complex, time-consuming, and expensive. It is relatively easy and inexpensive to test for coliform bacteria. Total coliform testing is used as an indicator of potential contamination.
Total Coliform, Fecal Coliform, and E. 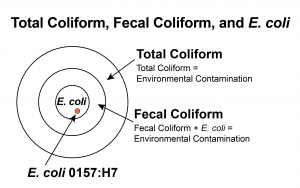 coli – Total coliform bacteria are commonly found in the environment (e.g., soil or vegetation) and are generally not harmful. However, if environmental contamination can enter the system, there may also be a way for pathogens to enter the system.
coli – Total coliform bacteria are commonly found in the environment (e.g., soil or vegetation) and are generally not harmful. However, if environmental contamination can enter the system, there may also be a way for pathogens to enter the system.
Fecal coliform bacteria are a sub-group of total coliform bacteria. They appear in great quantities in the intestines and feces of people and animals. The presence of fecal coliform in a drinking water sample often indicates recent fecal contamination, meaning that there is a greater risk that pathogens are present than if only total coliform bacteria is detected.
E. coli is a sub-group of the fecal coliform group. The presence of E. coli in a drinking water sample almost always indicates recent fecal contamination, meaning there is a greater risk that pathogens are present.
Responses to Coliform Detection – When coliform bacteria are found, water systems investigate to find out how the contamination got into the water. They collect additional, or “repeat,” water samples for testing, and often inspect the entire system. Taking repeat samples helps determine whether an actual problem exists in the system. If any of the repeat samples detect coliform bacteria, the initial findings are considered confirmed. If total coliform bacteria are confirmed in your drinking water, your water system should be inspected to find and eliminate any possible sources of contamination. Once the source is identified, it can usually be resolved by making system repairs, flushing, and adding chlorine for a short period of time.
Confirmation of fecal coliform bacteria or E. coli in a water system indicates recent fecal contamination, which may pose an immediate health risk to anyone consuming the water. Public notification, in the form of a boil advisory or potentially a do not drink advisory, will be issued within 24 hours to alert all water users that there is a health risk associated with the water supply. The notice will inform customers of actions being taken to correct the problem, and when the problem will likely be resolved.
The system will be required to identify the source of contamination, correct the problem, and thoroughly disinfect and flush its system. Once the contamination issue is verified to be resolved the advisory will be lifted.
Health Effects – Symptoms of bacterial waterborne diseases may include gastrointestinal illnesses such as severe diarrhea, nausea, and possibly jaundice as well as minor symptoms like headaches and fatigue. These symptoms are not associated only with disease-causing organisms in drinking water and may be caused by several other factors. Young children and the elderly are usually more susceptible.
Additional Information
Fluoride is a naturally occurring compound derived from fluorine—the earth’s 13th most abundant element. It is found in many rocks and minerals and can enter drinking water as water passes through soil. Fluoride is naturally present in almost all foods and beverages, including water. Only one public water system in Idaho currently adds fluoride to the drinking water. All other reported fluoride levels in Idaho’s public water systems are from naturally occurring fluoride backgrounds.
Fluoride Regulation – Fluoride has been shown to prevent tooth decay, but too much fluoride at an early age can cause discoloration and pitting of the teeth. This condition is known as dental fluorosis. Overexposure to fluoride over a lifetime can lead to certain bone diseases.
EPA has set a maximum contaminant level (MCL) for drinking water from community public water systems. People who regularly consume water containing fluoride concentrations above the MCL may experience bone disease. There is no fluoride drinking water standard for non-community public water systems
EPA has also set a secondary standard of 2.0 mg/L and recommends that children under nine years old not regularly consume water with fluoride concentrations higher than this standard. Children who regularly consume water above this level may experience dental fluorosis, ranging from white flecks to brown stains and pitting. The U.S. Department of Health and Human Services (HHS) recommends that drinking water contain 0.7 mg/L of fluoride for optimal oral health. Your dentist can help determine how much additional fluoride, if any, your family and you need.
Testing for Fluoride – Several methods are available to determine fluoride concentrations:
- If your water comes from a public water system, ask your water provider. If they are unable to locate the results, download them from the DEQ’s Drinking Water Watch.
- If you have a private well, have your water tested by a qualified laboratory to determine fluoride concentrations. Your local public health district can provide information on sampling and testing your drinking water. Fluoride levels in drinking water can fluctuate naturally, so a sample may not represent a constant concentration.
If you have been advised by a professional that the concentration of fluoride in your drinking water is too high, it may be necessary to drink bottled water, find an alternate source of potable water, or install treatment on your water source.
Reducing Fluoride in Your Drinking Water – Community water systems that exceed the MCL are required to reduce fluoride levels. The best available technologies for control of fluoride in drinking water are reverse osmosis or activated alumina. Other treatment options for fluoride removal include adsorptive media, chemical treatment, ion exchange, membrane separation, and an electrocoagulation process.
All regulated public water systems must have a DEQ-approved preliminary engineering report and DEQ-approved plans and specifications before construction, modification, or installation of any drinking water treatment processes.
Home water treatment systems are also available to remove fluoride from drinking water. Point-of-use distillation and reverse osmosis are treatment methods that have proven to be effective for removing fluoride. The typical charcoal-based water filtration systems (e.g., pitcher-type filters) do not remove fluoride from water. Boiling water also does not remove fluoride.
Only water used for drinking or cooking needs to be treated when fluoride concentrations exceed the MCL or secondary standard because fluoride is not absorbed through the skin. DEQ recommends ensuring all treatment system components and chemicals are certified by NSF. Always follow the manufacturer’s recommendations for the operation and maintenance of any water treatment system. Occasional sampling of water produced by the treatment system is also recommended to determine the effectiveness of the treatment.
Additional Resources
Lead is a toxic metal that was commonly used in consumer products such as gasoline and paint before it was discovered that it is harmful to human health. We now know that, if inhaled or swallowed, lead can build up in the body over time and cause serious damage to the brain, kidneys, nervous system, and red blood cells.
The problem of airborne lead exposure has been largely resolved in the United States as a result of the phase-out of leaded gasoline. Today, exposure is more likely to come from lead-contaminated soil or dust and drinking water through the corrosion of plumbing.
Impacts of Lead Exposure – Exposure to lead is most dangerous for young children under the age of six and infants. A dose of lead that would have little effect on an adult could have a big effect on a small body. The primary sources of lead exposure for most children are deteriorating lead-based paint, lead-contaminated dust, and lead-contaminated residential soil. On average, it is estimated that lead in drinking water accounts for 10% to 20% of total lead exposure in young children. Lead exposure in drinking water may be as high as 60% in infants whose diet consists mostly of liquids made with lead-contaminated water.
Lead and Drinking Water – Certain drinking water plumbing, older fixtures, or solder may contain lead. The most common cause of lead in drinking water is corrosion, a reaction between water and lead pipes or solder. Lead may be present in your drinking water if your home has faucets or brass fittings that contain lead, or if your home or water system (service line and internal plumbing) has lead or copper pipes with solder that contains lead. Lead can be present in school drinking water as well, particularly when water sits overnight, over a weekend, or during a vacation. The lead concentration depends on the plumbing materials and the corrosivity of the water.
On July 29, 2020, the U.S. Environmental Protection Agency (EPA) issued the final regulation “Use of Lead-Free Pipes, Fittings, Fixtures, Solder, and Flux for Drinking Water.” In the final rule, EPA makes conforming changes to existing regulations based on the Reduction of Lead in Drinking Water Act (RLDWA) and the Community Fire Safety Act enacted by Congress. The final rule also requires that manufacturers or importers certify that their products meet the requirements using a consistent verification process within 3 years of the final rule publication date in the Federal Register. As a result, this new rule will reduce lead in drinking water and assure that states, manufacturers, inspectors and consumers have a common understanding of “Lead-Free” plumbing.
How to Reduce Exposure to Lead in Drinking Water
Flush pipes before drinking – The longer water stands idle in plumbing, the more lead it may absorb. Do not drink or cook with water that has been idle in your plumbing for more than 6 hours (e.g., overnight or during the workday). Flush your pipes by running the cold water for at least 30 to 60 seconds. To prevent wasting flushed water, use it for watering plants or washing dishes. Boiling water concentrates lead and does not remove it.
Consume cold water only – Hot water is likely to contain higher levels of lead. If you need hot water for cooking or drinking, use cold water and heat it. Do not use hot tap water to make baby formula.
Have the water tested – Because you cannot see, taste, or smell lead dissolved in water, the only way to ensure your household water does not contain harmful quantities of lead is to have it tested by a laboratory. Closely follow sampling instructions outlined by the laboratory to avoid tainting results. Contact your public water system or health department for information and assistance.
Some treatment devices can reduce the amount of lead in drinking water. All treatment devices should be certified by the National Sanitation Foundation.
Lead and Copper Site Selection – The Lead and Copper Rule (LCR) requires all community and non-transient, non-community public water systems to sample at locations that may be particularly susceptible to high lead or copper concentrations per the “Rules for Public Drinking Water Systems” (IDAPA 58.01.08.350.07).
The LCR establishes a tiering system for prioritizing sampling sites based on a materials evaluation. Most existing water systems conducted this survey in 1992 but would benefit from conducting the survey again as materials may have changed. New water system owners and operators must perform a materials evaluation before lead and copper tap monitoring. The Lead and Copper Sample Site Selection form defines the monitoring requirements and tiering system for prioritizing sampling sites and includes a site selection certification form for submittal to DEQ.
Proposed Revisions to the Lead and Copper Rule – In 2019, EPA published proposed revisions to the Lead and Copper Rule that include a suite of actions to reduce lead exposure in drinking water. The proposed rule will identify the most at-risk communities and ensure systems have plans in place to rapidly respond by taking actions to reduce elevated levels of lead in drinking water.
The agency’s proposal takes a proactive approach to improving the current rule, including testing, treatment, and informing the public about the levels and risks of lead in drinking water.
This approach focuses on six key areas:
- Identifying the areas most impacted.
- Strengthening drinking water treatment requirements.
- Replacing lead service lines.
- Increasing sampling reliability.
- Improving risk communication.
- Protecting children in schools and childcare facilities.
Learn more about proposed visions for the LCR at https://www.epa.gov/ground-water-and-drinking-water/proposed-revisions-lead-and-copper-rule.
Additional Resources
Nitrate is an inorganic chemical contaminant common to drinking water systems in Idaho. High levels of nitrate can cause serious health effects, especially for young children. Nitrate comes from septic and sewer systems, waste from animal feedlots, nitrogen-based fertilizers, and natural deposits. Runoff from irrigation, flooding, and precipitation often leads to seasonal peaks in drinking water nitrate levels.
Private drinking water well owners should test for nitrate at least annually. All public water systems are required to monitor for nitrate contamination regularly. Each state determines the frequency of monitoring based primarily on the most recent testing results and available historic data, although factors affecting source water quality and susceptibility may be considered as well. Systems that do not meet EPA’s established Maximum Contaminant Level (MCL) must provide treatment to protect the health of those it serves.
Regulatory Protections – The Maximum Contaminant Level (MCL) is the maximum permissible level of a contaminant in water that is delivered to any user of a public water system. The MCL for nitrate is 10 mg/L. Public drinking water systems with sample results that are equal to or greater than the ½ MCL of 5 mg/L must monitor quarterly until four consecutive samples are reliably and consistently below the MCL. Systems with sample results that are equal to or greater than the MCL of 10 mg/L must take a second sample to confirm the result. If the average of the two samples does not meet requirements the system must sample quarterly and inform its users through public notification until the problem is resolved.
Health Effects – Nitrate exposure can have serious health effects, but certain groups are particularly vulnerable, including people with pre-existing health conditions, pregnant women, and young children under the age of six months. Infants who drink water containing nitrate above the MCL can become seriously ill and, if untreated, may die. Symptoms include shortness of breath and blue-baby syndrome, also known as Methemoglobinemia. This condition can occur rapidly, over the course of just a few days. Do not attempt to remove nitrate from drinking water by boiling it. This will concentrate nitrate levels.
Private Wells – It is the well owner’s responsibility to maintain the well and ensure that the water is safe to drink. Private well owners should test for nitrate at least annually. More information on installation, maintenance, and testing can be found on our Ground Water Well web page.
Additional Resources – Each owner or operator of a public water system must notify customers of any national primary drinking water regulations violations. Notifications must include information about the health effects of the contaminant and how they may be mitigated or prevented. Pay attention to public notices and defer to the system’s Consumer Confidence Report (CCR) for more information. Review the contact information listed on the monthly water bill or contact your DEQ regional office for assistance.
The purpose of the disinfection byproducts (DBP) rules is to reduce potential cancer, reproductive, and developmental health risks from disinfection byproducts in drinking water, which form when disinfectants are used to control microbial pathogens.
Stage 1 DBP rule – This rule applies to all community and non-transient non-community public water systems that use a disinfectant for either primary or residual water treatment. Transient water systems that apply chlorine dioxide as a disinfectant must also comply.
- EPA website reference: Stage 1 Disinfectant and Disinfection Byproduct Rule
- DEQ resource: Implementation Guidance for the Stage 1 Disinfectants and Disinfection By-Products Rule
Stage 2 DBP rule – This rule applies to community and non-transient non-community water systems that produce and/or deliver water that is treated with a primary or residual disinfectant other than ultraviolet light. The Stage 2 DBP rule also covers consecutive systems. Consecutive systems are public water systems that receive some or all of their finished water from one or more wholesale systems.
- EPA website reference: Stage 2 Disinfectant and Disinfection Byproduct Rule
- DEQ resource: Implementation Guidance for the Stage 2 Disinfectants and Disinfection By-Products Rule
- EPA website reference: Compliance Help: Stage 2 Disinfectants and Disinfection Byproducts Rule
Includes quick reference guides, fact sheets, guidance manuals, and training links as well as guidance for small systems that serve fewer than 10,000 people. - EPA resource: Complying with the Stage 2 Disinfectant and Disinfection Byproducts Rule: Small Entity Compliance Guide—One of the Simple Tools for Effective Performance (STEP) Guide Series
Monitoring Requirements under the Stage 2 DBP Rule
Compliance monitoring plans – Owners and operators of systems subject to the Stage 2 DBP must develop a Compliance Monitoring Plan (CMP) identifying how they intend to sample for compliance. Systems must prepare a plan before they begin their Stage 2 DBP compliance monitoring and must keep the plan on file. Surface water (SW) systems and ground water under the influence (GWUDI) of surface water systems with populations over 3,300 must submit their CMPs to the state for review (unless a system prepared an Initial Distribution System Evaluation (IDSE), which were required prior to 2012, report and the IDSE report already contains the required information).
Example Plans:
- SW system serving less than 500 people
- SW system serving 500–3,300 people
- SW system serving 3,301–9,999 people
- SW system serving 10,000–49,999 people
- GW system serving less than 500 people
- GW system serving 500–9,999 people
- GW system serving 10,000–49,999
Routine Monitoring – Routine monitoring must be conducted according to the information in the following table:
- Systems that are on Stage 1 DBP increased monitoring must begin Stage 2 DBP compliance monitoring on increased monitoring.
- Systems that are on Stage 1 DPB reduced monitoring may begin Stage 2 DBP compliance monitoring on reduced monitoring if all the requirements have been met.
Reduced Monitoring – Reduced monitoring must be conducted according to the information in the following table:
Systems that are on reduced Stage 1 DBP monitoring may remain on reduced monitoring for Stage 2 DBP if all of the following criteria are met:
- The system qualifies for a 40/30 certification or received a Very Small System (VSS) waiver.
- The monitoring locations for Stage 1 DBP and Stage 2 DBP are the same.
- The average of all samples taken in the year prior to starting Stage 2 DBP compliance monitoring is no more than 0.040 milligrams per liter (mg/L) for total trihalomethanes (TTHM) and no more than 0.030 mg/L for haloacetic acids (HAA5).
Systems that are on routine Stage 2 DBP monitoring may qualify for reduced monitoring as determined by DEQ if the following criterion is met:
- The locational running annual average (LRAA) of all samples taken for 1 year after Stage 2 DBP compliance monitoring starts is no more than 0.040 mg/L for TTHM and no more than 0.030 mg/L for HAA5.
Systems that are on reduced Stage 2 DBP monitoring may remain on reduced monitoring if the following criterion is met:
- For systems that are on yearly or less frequent (3 years) monitoring, the LRAA of all samples is no more than 0.060 mg/L for TTHM and no more than 0.045 mg/L for HAA5.
In addition to the criteria listed above for systems using surface water or ground water under the direct influence, the running annual average (RAA) for total organic carbon (TOC) level must be below <4.0 mg/L at each treatment plant based on monitoring conducted under Stage 1 DBP rule.
Note that an LRAA is calculated using four quarters of data. If a water system exceeds the LRAA criteria at any location, the system does not meet the reduced monitoring requirements. If monitoring results indicate that a system is no longer eligible for reduced monitoring, the system must resume routine monitoring or be placed on increased monitoring.
Increased Monitoring – Systems on an increased Stage 1 DBP monitoring schedule must begin Stage 2 monitoring on the increased schedule until they meet the requirements for returning to the routine schedule.
Systems on Stage 2 DBP that are on annual or less frequent monitoring must go to increased monitoring if any sample at any location exceeds the maximum contaminant level (MCL) for either TTHM (0.080 mg/L) or HAA5 (0.060 mg/L). The system must increase monitoring frequency to dual sample sets once per quarter (taken every 90 days) at all locations.
Systems on Stage 2 DBP that are on increased monitoring can return to routine monitoring if, after four quarters of consecutive monitoring, each monitoring location’s LRAA for TTHM or HAA5 is less than 0.060 mg/L or 0.045 mg/L, respectively.
Note that increased monitoring is not based on an LRAA but rather on individual sample results. If any sample at any location is above either of the MCLs, then increased monitoring applies system-wide, not just at one location.
Calculation of TTHM and HAA5 under the Stage 2 DBP Rule
Locational Running Annual Average – Compliance with the TTHM and HAA5 MCLs for Stage 2 DBP rule is based on the monitoring results and locational running annual average (LRAA) calculations at each monitoring location as shown in the following example:
TTHM and HAAS Compliance Requirements
| TTHM and HAAS compliance | Quarterly monitoring | Once per year or less frequent monitoring |
|---|---|---|
| Is based on | LRAA-calculated quarterly for each sampling location | Value of yearly or less frequent samples at each sampling location |
| If LRAA exceeds the MCL | Violation if any sampling point exceeds the LRAA* | Not immediately in violation; start quarterly monitoring to determine compliance |
*Compliance is based on the LRAA so if any one sample exceeds an annual average (e.g., over four times the MCL) or any combination of samples in the four quarters exceeds the MCL, the system is in violation of the MCL.
For quarterly monitoring:
- If the system fails to complete four consecutive quarters of monitoring, compliance with the MCL will be calculated based on the average of the available data from the most recent four quarters.
- If the system takes more than one sample per quarter at a monitoring location, all samples taken in the quarter at that location will be averaged to determine a quarterly average to be used in the LRAA calculation.
Operation Evaluation Levels – As a part of Stage 2 DBP rule compliance monitoring, owners and operators of systems are required to calculate operation evaluation levels (OELs) by calculating the sum of the two previous quarters’ results plus twice the current quarter’s results divided by 4 as shown in the following example:
This calculated LRAA result should help water systems identify if they are likely to exceed the MCL in the next quarter, and give them a chance to make operational changes.
If OELs are higher than the MCL for TTHM and HAA5 at any location in the distribution system, the owner or operator of the system needs to conduct an operational evaluation and submit a report to DEQ. If the system is able to readily identify the cause of the OEL exceedance, the operational evaluation report may be limited.
- Operation Evaluation Level (OEL) Reporting Form
- EPA website reference: Operational Evaluation Guidance Manual
Additional Information
The purpose of the Long Term 2 Enhanced Surface Water Treatment Rule (LT2ESWTR) is to reduce illness linked with the contaminant Cryptosporidium and other microbial pathogens in drinking water. The LT2ESWTR supplements existing regulations for surface water systems, including ground water under the direct influence of surface water (GWUDI) systems, by targeting additional Cryptosporidium treatment requirements for systems with higher risk sources found during LT2ESWTR monitoring. It is important to note that the LT2ESWTR does not require additional Cryptosporidium treatment for all public water systems (PWS). The LT2ESWTR also contains provisions to reduce risks resulting from uncovered finished water reservoirs and to ensure that systems maintain microbial protection as they take steps to decrease the formation of disinfection byproducts that result from chemical water treatment.
Key Provisions
- The LT2ESWTR applies to all PWS supplied by a surface water or GWUDI source.
- All surface water and GWUDI systems must conduct an initial round of source water monitoring and a second round 6 years later for each plant that treats a surface water or GWUDI source. This monitoring includes coli enumeration (and turbidity if required) to determine what level, if any, of additional treatment must be provided.
- Filtered systems must determine their Cryptosporidium treatment bin classification and provide additional treatment for Cryptosporidium, if required.
- All unfiltered systems must provide treatment for Cryptosporidium.
General Source Water Monitoring Information – Any new surface water or GWUDI sources must conduct an initial and a second round of source water monitoring.
Filtered systems serving fewer than 10,000 people must sample their source water (prior to any treatment) for E. coli enumeration (not presence/absence) at least once every two weeks for 12 months.
- Afiltered system serving fewer than 10,000 people may avoid coli monitoring by monitoring for Cryptosporidium and notifying DEQ of this no later than 3 months prior to the date the system is otherwise required to start E. coli monitoring.
- Filtered systems serving fewer than 10,000 people must sample their source water (prior to any treatment) for Cryptosporidium at least twice per month for 12 months or at least monthly for 24 months based on the annual mean coliconcentration of their source water type (lake/reservoir vs. flowing stream).
Systems using GWUDI must also sample their  source water (prior to any treatment) for E. coli at least once every two weeks for 12 months based on the E. Coli level that applies to the nearest surface water body. If no surface water body is nearby, the system must comply based on the requirements that apply to systems using lake/reservoir sources.
source water (prior to any treatment) for E. coli at least once every two weeks for 12 months based on the E. Coli level that applies to the nearest surface water body. If no surface water body is nearby, the system must comply based on the requirements that apply to systems using lake/reservoir sources.
Sample Schedule Plans – Prior to sampling, owners and operators must submit to DEQ a sampling plan that includes the following:
- Specific dates for sampling events that must occur at least once every 2 weeks for 12 months for systems serving less than 10,000 people and monitoring for coli, beginning October 1, 2017.
- Samples must be collected within 2 days before or 2 days after the specified date (Wednesdays are recommended), and the results must be submitted to DEQ by the 10th day of the following month.
- Description or diagram of sample location(s) in relation to sources, treatment processes, (including pretreatment), and any filter backwash recycling.
Sample Specific Sampling Requirements – Systems with more than one surface water supply source, one of the following is required for collecting samples:
- Collect samples from a tap where all sources are combined and before any treatment.
- Collect composite samples that represent all sources. In a composite sample, the volume of sample from each source must be weighted according to the proportion of each source in the total plant flow at the time of the sample. You should contact DEQ and your laboratory for assistance.
- Collect and analyze a sample from each source separately and calculate a weighted average of all the results for the sampling date. You should contact DEQ and your laboratory for assistance.
Seasonal Sources
- Owners and operators of systems with seasonal sources need to sample in the months of operation, which is when the plant is running, not only when water is being served.
- If the system operates fewer than 6 months a year, two sampling periods, with six evenly spaced samples in each period, must take place over the course of 2 seasons. For example, if a system operates May through September, they would sample May through September 2017 and again in May – September 2018).
- If the system operates ≥ 6 months but less than a full year, two sampling periods during months of operation must take place and samples must be taken at least once every 2 weeks.
Systems Using Bank Fileration
- Systems using bank filtration followed by a filtration plant must collect samples from the well and after bank filtration.
- Systems receiving Cryptosporidium treatment credit for bank filtration must collect source water samples in the surface water before bank filtration.
If conditions pose a danger to someone collecting samples or are unforeseen and could not be avoided that prevent a sample from being collected, the operator must collect a sample as close to the scheduled date as possible and submit an explanation for the new sampling date along with the laboratory results.
Sample Collection and Submission
- EPA Crypto and Sample Collection Recommendations – Pocket Guide
- EPA LT2ESWTR Source Water Monitoring Guidance
**NOTE – Samples must be maintained at 0–10 oC (32–50 oF), but not frozen, during shipment to the laboratory for E.coli enumeration analysis. Samples received outside this temperature range will be rejected.
Bin Calculation for Filtered Systems – Following the completion of the second round of source water monitoring, DEQ will recalculate each PWS’s Cryptosporidium bin concentration using the results reported.
- Step #1 – The results of all the samples for 12 months (one sample at least every two weeks) are added together.
- Step #2 – The sum of all the samples from Step #1 is divided by the total number of samples to determine the annual mean.
Under LT2ESWTR, DEQ may approve an alternative to the small water system E. coli trigger levels (currently 10 E. coli/100ml for lake/reservoir or less than 50 E.coli/100ml for flowing stream sources.
According to EPA “analysis indicates 100 E. coli/100ml for lake/reservoir AND flowing streams provide more accurate identification of systems requiring Crypto monitoring and compliance with LT2 treatment technique requirements.” Idaho has adopted this approach of using 100 E. coli/100 ml.
Additional Resources
- Implementation Guidance for the LT2EWSTR
- LT2ESWTR E.coli Sample Information Required for Lab Analysis from PWS
- Summary of E.coli Enumeration Sampling Collection Under LT2ESWTR
- Idaho Drinking Water Labs Certified for LT2ESWTR
- EPA LT2ESWTR webpage
- EPA LT2ESWTR Source Water Monitoring Guidance
- EPA LT2ESWTR Source Water Monitoring Factsheet for PWSs serving <10,000
- EPA LT2ESWTR Small Entity STEP Guide
- EPA Crypto and coli Sampling Recommendations – Pocket Guide
Unregulated Contaminants
- One-day and 10-day health advisories are considered acute or short-term levels that are not expected to cause adverse effects for up to one or ten days of exposure. These health advisories are intended to protect a 10-kg (22 pound) child consuming 1 liter of water per day.
- Lifetime health advisories are considered chronic or long-term levels that are not expected to cause adverse effects after a lifetime of exposure. These health advisories are intended to protect a 70-kg (154 pound) adult consuming 2 liters of water per day.
This accordion will not appear on the screen
Cyanobacteria, often referred to as blue-green algae, are bacteria that photosynthesize like algae and plants. Cyanobacteria naturally occur in freshwater environments but, under certain conditions, can reproduce rapidly and form floating mats and dense surface scums, commonly referred to as harmful algae blooms (HABs). HABs produce cyanotoxins, which can pose a risk to human health.
EPA is determining whether to regulate cyanotoxins in drinking water due to updated health information and occurrence data. EPA included several types of cyanotoxins in their fourth Unregulated Contaminant Monitoring Rule (UCMR4), which requires all public water systems utilizing surface water or ground water influenced by surface water and serving over 10,000 customers to monitor for certain cyanotoxins. UCMR4 also applies to some smaller systems.
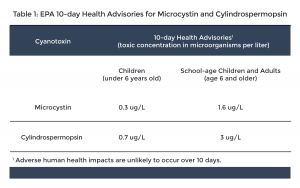 Cyanotoxin health advisory levels – In 2015, EPA established health advisory levels for two cyanotoxins: microcystin and cylindrospermopsin. Health advisory levels are recommended contaminant levels meant to provide a margin of protection for all water system users based on a specified exposure time from adverse health effects resulting from the contaminant. When these toxins are found in drinking water, the concentrations should be compared with the health advisory levels.
Cyanotoxin health advisory levels – In 2015, EPA established health advisory levels for two cyanotoxins: microcystin and cylindrospermopsin. Health advisory levels are recommended contaminant levels meant to provide a margin of protection for all water system users based on a specified exposure time from adverse health effects resulting from the contaminant. When these toxins are found in drinking water, the concentrations should be compared with the health advisory levels.
See EPA’s website for health advisory information for cyanotoxins.
Health effects of cyanotoxins – Adverse health effects from cyanotoxins depend on many factors, including the type of cyanotoxin(s) present, duration of exposure, and the age and health of the individual. Cyanotoxins may affect the liver, nervous system, or skin, depending on the toxin. Health effects from cyanotoxin exposure range from skin rashes to liver and nerve damage. No human deaths in the United States have been caused by cyanotoxins, however, pet, livestock, and wildlife deaths caused by cyanotoxins have been reported throughout the United States and the world.
If you concerned about your health or have symptoms, contact your healthcare provider.
Public water systems monitoring for cyanotoxins in Idaho – Public water systems are not required to monitor for cyanotoxins. Some systems may voluntarily monitor for cyanotoxins. Contact your public water system to find out if they monitor for cyanotoxins.
Removing cyanotoxins from water – Boiling water will not remove cyanotoxins and will concentrate the toxins.
If your drinking water has been impacted by cyanotoxins and a “Do Not Use” or a “Do Not Drink” order has been issued, use an alternative source of water for drinking.
Point-of-use devices can be used to reduce levels of microcystins. National Sanitation Foundation International developed NSF Protocol 477: Drinking Water Treatment Units – Microcystin that verifies a water filter’s ability to reduce microcystin below the health advisory levels set by EPA.
Treatment devices require regular maintenance such as changing filters, cleaning scale buildup, or disinfecting the unit. Failure to properly maintain a unit reduces its effectiveness and, in some cases, may further impair the water quality. Follow the manufacturer’s recommendations for replacements and maintenance.
Once a cyanotoxin-related health advisory is lifted, you should flush all of your household plumbing and replace all water filters in the house to remove toxins:
- Run and flush hot water taps for 15 minutes and all cold water taps for 5 minutes.
- Change all filters on point of use units, point of entry systems, refrigerator water filters, and membranes for reverse osmosis units.
- Ensure all faucets and plumbed appliances have been flushed.
- Additional flushing instructions.
Additional Resources
Manganese in Drinking Water
Manganese is a common and naturally occurring metal found in over 100 minerals. It can be found in soil, air, water, and foods such as nuts, grains, fruits, tea, leafy vegetables, some infant formulas, and some meat and fish. It is used in the manufacturing of iron and steel alloys and as a component in batteries, glass, gasoline, fertilizers, and fireworks.
Manganese is an essential nutrient for humans and animals and adverse health effects can occur with too little or too much manganese. Adults and children are primarily exposed to manganese through food. Drinking water is also a source of manganese but normally in lower amounts. Air exposure is less common but can vary depending on proximity to industrial sources.
Is manganese regulated? Manganese is not a regulated contaminant in drinking water nationally, though some states have set their own standards. Idaho has not adopted a drinking water standard for manganese.
Manganese is a drinking water secondary contaminant, meaning water that is over the secondary standard of 0.05 mg/L is known to cause cosmetic or aesthetic effects such as a metallic taste, stained plumbing fixtures, and discolored water.
EPA is currently determining whether to regulate manganese due to updated health effect information and occurrence data. EPA included manganese in the fourth Unregulated Contaminant Monitoring Rule (UCMR4), which required all public drinking water systems serving over 10,000 people and selected small systems to monitor for manganese. EPA will also consider the health effects in its regulatory determination and evaluate potential risks to children and infants based on recent studies (81 FR 81099).
For more information visit EPA’s regulatory determination process web page.
For more information on the UCMR4 can be found on EPA’s website.
What are the health advisory levels? Health advisories provide information on contaminants that can cause human health effects and are known (or anticipated) to occur in drinking water. Health advisories are intended to provide technical guidance to agencies and local officials. In 2004, EPA issued a drinking water health advisory for manganese. EPA’s health advisory information for manganese can be found here.
Health Advisory Levels: 0.3 milligrams per liter (mg/L) and 1 mg/L – Infants younger than six months should not consume water that contains manganese over 0.3 mg/L. Some baby formulas contain manganese as a nutrient and should not be prepared with water that also contains manganese.
EPA established a one-day and ten-day short-term (acute) advisory at 1 mg/L for adults and children. This advisory identifies the concentrations below which potential health problems would unlikely occur for healthy individuals over six months old.
EPA also established a lifetime health advisory at 0.3 mg/L. Lifetime health advisories are considered chronic or long-term levels that are not expected to cause adverse effects after a lifetime of exposure. These health advisories are intended to protect a 70-kg (154 pound) adult consuming two liters of water per day.
Public Notification Requirement for Manganese – DEQ requires that public drinking water systems provide immediate notification to their customers when manganese results exceed the short term health advisory of 0.3 mg/L. DEQ has the authority to require public notification for situations with significant potential to have serious adverse effects on human health as a result of short-term exposure (Idaho Rules for Public Water Systems, IDAPA 58.01.08.150.02, which incorporates 40 CFR 141.202(a)(9)).
What are the health effects of manganese? Adverse health effects from too much manganese depend on many individual factors, including manganese consumption, age, current health conditions, diet, and nutritional status. Too much manganese can harm the nervous system, resulting in behavioral changes and other nervous system effects, including slow and clumsy movements. Some studies have shown that too much manganese during childhood may also have effects on the brain, which may affect learning and behavior.
Some people may be more sensitive to manganese, including bottle-fed infants under six months, as indicated by the short-term health advisory, as well as the elderly and those with liver disease.
If you are concerned about your health from manganese exposure, contact your healthcare provider.
Do public water systems monitor for manganese? Since manganese is not regulated by EPA as a primary drinking water contaminant, monitoring for manganese in Idaho public drinking water systems is not required unless the system is part of UCMR4, which requires all public drinking water systems serving over 10,000 people and selected small systems to monitor for manganese. Some systems may voluntarily monitor for manganese.
Contact your public water system to find out if they test for manganese. If your provider does not test for manganese or you have a private well, you can pay a certified laboratory to test your water. Certified laboratories are listed on the Idaho Bureau of Laboratories website.
How do I remove manganese from my water? Do not boil your water. Boiling water increases manganese concentration; it does not remove it.
Filter your drinking water or use an alternate source if you are concerned. Oxidizing filters, reverse osmosis units, or water softeners can lower manganese levels in tap water, depending on the form of manganese in your water (dissolved or particulate). National Sanitation Foundation International, the Water Quality Association, Underwriters Laboratories, and Canadian Standards Association International all certify home water treatment products for contaminant removal. However, current third-party certifications do not address manufacturer claims for home treatment device manganese reduction.
Treatment devices require regular maintenance such as changing filters, cleaning scale buildup, and disinfecting the unit. Failure to properly maintain a unit reduces its effectiveness and, in some cases, may exacerbate water quality issues. Follow manufacturer recommendations for filter replacements and maintenance.
In some cases, purchasing bottled water may help reduce your exposure to manganese, though manganese may still be present in bottled water. Contact the bottled water manufacturer for more water quality information.
Additional Resources
Per- and polyfluoroalkyl substances (PFAS) are a group of more than 4,000 man-made chemicals, including perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS), that have been used worldwide since the 1940s.
PFAS are fluorinated organic chemicals that do not occur naturally in the environment. These chemicals are found in common household products such as cookware, carpets, upholstered furniture, mattresses, clothing, food packaging, cosmetics, coated paper, and products treated with stain, soil, or water repellants. They are also used for firefighting at airfields and in many industrial processes. Due to widespread use, most people have been exposed to these chemicals.
Contamination is typically localized and associated with an industrial facility where these chemicals were produced or used in manufacturing, fire training facilities, landfills, wastewater treatment plants, or at an airfield where they were used for firefighting.
Health Advisory Level—On June 15, 2022, EPA replaced the 2016 health advisory level of 70 parts per trillion (ppt) with interim lifetime health advisory levels for PFOA at 0.004 ppt and PFOS at 0.02 ppt. EPA also established final lifetime health advisory levels for hexafluoropropylene oxide (HFPO) (also known as GenX) at 10 ppt and perfluorobutane sulfonic acid (PFBS) at 2,000 ppt. GenX is considered a replacement for PFOA, and PFBS is considered a replacement for PFOS. Refer to EPA’s health advisory level for additional information.
Do public water systems monitor for PFAS? Idaho adopts EPA’s national primary drinking water standards, and currently, PFAS are not regulated contaminants. This means that public drinking water systems are not required to monitor for these contaminants.
Some public water systems in Idaho participated in EPA’s third round of the Unregulated Contaminant Monitoring Program (UCMR3) from 2013 to 2015, and others voluntarily collected samples that included testing for PFOA, PFOS, GenX, and PFBS. EPA’s website provides information and results from UCMR3. Many systems in Idaho will participate in EPA’s fifth round of the Unregulated Contaminant Monitoring Program (UCMR5) beginning in 2023 and ending in 2025, which includes PFAS contaminants. Find more information on EPA’s website at UCMR5.
What are the health effects of PFAS? Adverse health effects from PFAS (PFOA or PFOS) depend on the level, length of exposure, and an individual’s age, lifestyle, and health. PFAS contaminants can cause a variety of health effects:
- Developmental effects to fetuses during pregnancy or to breastfed infants (e.g., low birth weight, accelerated puberty, and skeletal variations)
- Cancer (e.g., testicular and kidney)
- Liver effects (e.g., tissue damage)
- Immune effects (e.g., antibody production and immunity)
- Thyroid effects
- Other effects (e.g., cholesterol changes)
EPA’s health advisories are set at levels meant to protect all people, including sensitive populations and life stages, from adverse health effects resulting from a lifetime exposure to PFAS in drinking water. EPA’s lifetime health advisories consider other potential sources of exposure to PFAS beyond drinking water (e.g., food, air, consumer products, etc.), which is meant to provide an additional layer of protection. Contact your healthcare provider if you have concerns or have symptoms you think may be caused by PFAS exposure.
How do I remove PFAS from my water? PFAS compounds cannot be removed by boiling water. If PFAS levels in your drinking water are above the health advisory levels, consider using alternate drinking water sources or installing a point-of-use treatment device.
Point-of-use units can be installed under a sink. Point-of-entry units can be installed at your home’s main water line. Reverse osmosis home filtration units have shown the greatest removal potential and granular-activated carbon may also be effective in removing PFAS.
Consider using units certified through third-party organizations that test and verify chemical reduction claims. These units will have a certification label from organizations such as the National Sanitation Foundation, Underwriters Laboratory, and Water Quality Association.
Any type of treatment device requires regular maintenance such as changing filters, cleaning scale buildup, or disinfecting the unit. Failure to properly maintain a unit reduces its effectiveness and, in some cases, may make the water quality worse. Continued maintenance is necessary for the life of the device along with regular water testing to ensure the device is working properly. Follow the manufacturer’s recommendations for replacement and maintenance.
Can I cook with PFAS contaminated water? If PFAS levels in your water are above the health advisory levels, consider using alternate sources of water for foods such as soups, rice, and beans where the water is absorbed. Tap water may be used to wash produce and dishes.
Can I use PFAS contaminated water for showering and bathing? PFAS compounds can enter the body through the skin. Bathing, swimming, and showering with water that has PFAS levels above the health advisory values could increase exposure.
Can I use PFAS contaminated water for laundry? Minimal traces of PFAS may remain on washed clothing and fabric. You can use water with PFAS levels above the health advisory values when washing clothing, bedding, and linens.
Can I use PFAS contaminated water in a humidifier? If the PFAS level in your water is above any of the health advisory levels, use distilled or treated water in your humidifier.
Can my pets drink PFAS contaminated water? The health effects on animals from PFAS exposure are likely similar to the effects on people. If PFAS levels in your water are above the health advisory levels or you are concerned about your pet’s health, contact your veterinarian.
Can I use the water for my garden? DEQ does not have adequate information to advise on this issue. Based on limited information, root vegetables and leafy vegetables may take in and build up PFAS from water and the soil.
Where do I get my water tested? Laboratories certified to test for PFAS (EPA Methods 533 and 537.1) are changing rapidly. Check laboratories certified to test for PFAS. Laboratories can only analyze PFAS samples if the box next to their name is marked with “X.” Costs for PFAS samples are estimated at $500–$600 per sample.
Additional Resources
This accordion will not appear on the screen
Decision Support Analyst, Drinking Water Protection
Drinking Water Analyst
Drinking Water Analyst

